Most Recent Publications
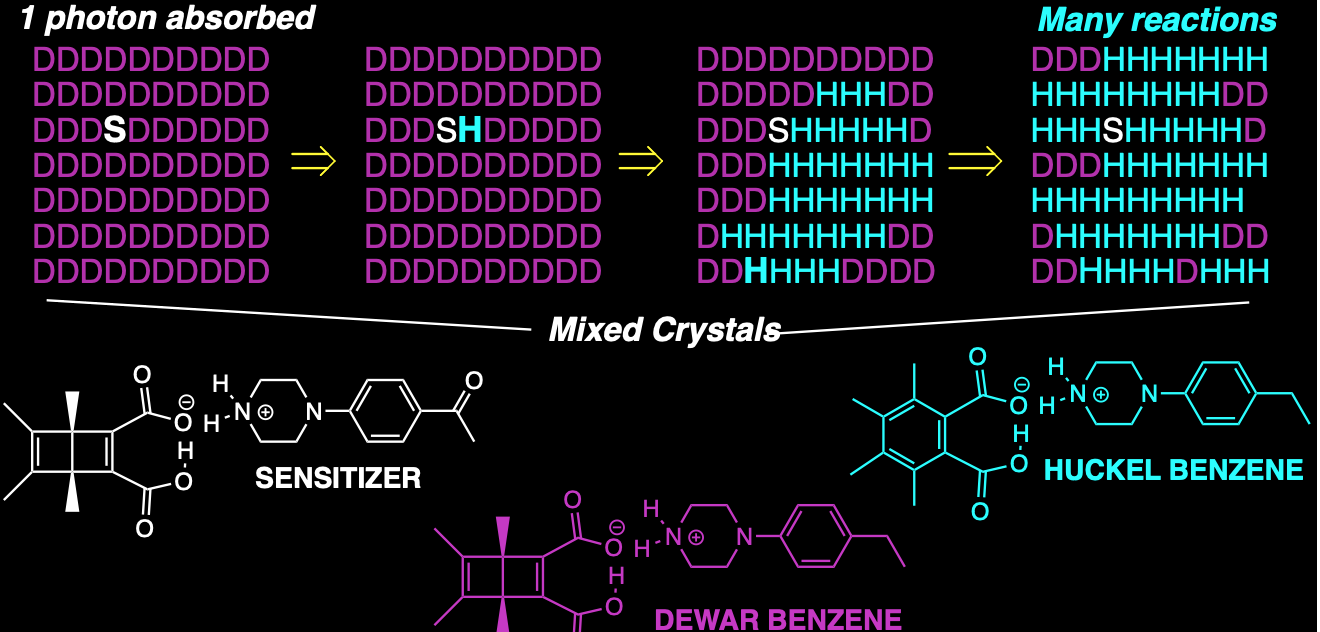
Reaction amplification with a gain: Triplet exciton–mediated quantum chain using mixed crystals with a tailor-made triplet sensitizer
Photochemical valence bond isomerization of a crystalline Dewar benzene (DB) diacid monoanion salt with an acetophenone-linked piperazinium cation that serves as an intramolecular triplet energy sensitizer (DB-AcPh-Pz) exhibits a quantum chain reaction with as many as 450 product molecules per photon absorbed (Φ ≈ 450). By contrast, isomorphous crystals of the DB diacid monosalt of an ethylbenzene-linked piperazinium (DB-EtPh-Pz) lacking a triplet sensitizer showed a less impressive quantum yield of ca. Φ ≈ 22. To establish the critical importance of a triplet excited state carrier in the adiabatic photochemical reaction we prepared mixed crystals with DB-AcPh-Pz as a dilute triplet sensitizer guest in crystals of DB-EtPh-Pz. As expected from their high structural similarities, solid solutions were easily formed with the triplet sensitizer salt in the range of 0.1 to 10%. Experiments carried out under conditions where light is absorbed by the triplet sensitizer-linked DB-AcPh-Pz can be used to initiate a triplet state adiabatic reaction from 3DB-AcPh-Pz to 3HB*-AcPh-Pz, which can serve as a chain carrier and transfer energy to an unreacted DB-EtPh-Pz where exciton delocalization in the crystalline solid solution can help carry out an efficient energy transfer and enable a quantum chain employing the photoproduct as a triplet chain carrier. Excitation of mixed crystals with as little as 0.1% triplet sensitizer resulted in an extraordinarily high quantum yield Φ ≈ 517.
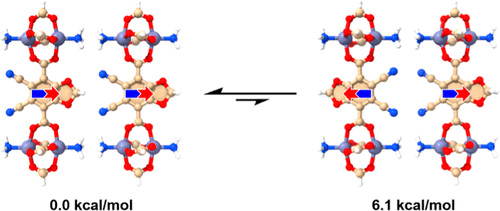
Computational Study of Ground-State Destabilization Effects and Dipole–Dipole Interaction Energies in Amphidynamic Crystals
Ground-state destabilization is a promising strategy to modulate rotational barriers in amphidynamic crystals. Density functional theory studies of polar phenylenes installed as rotators in pillared paddle-wheel metal organic frameworks were performed to investigate the effects of ground-state destabilization on their rotational dynamics. We found that as the steric size of phenylene substituents increases, the ground-state destabilization effect is also increased. Specifically, a significant destabilization of the ground-state energy occurred as the size of the substituents increased, with values ranging from 2 to 11.7 kcal/mol. An evaluation of the effects of substituents on dipole–dipole interaction energies and rotational barriers suggests that it should be possible to engineer amphidynamic crystals where the dipole–dipole interaction energy becomes comparable to the rotational barriers. Notably, while pure dipole–dipole interaction energies reached values ranging from 0.6 to 2.4 kcal/mol, the inclusion of electronic and steric effects can alter dipolar orientations to significantly greater values. We propose that careful selection of polar substituents with different sizes may help create temperature-responsive materials with switchable collective polarization.
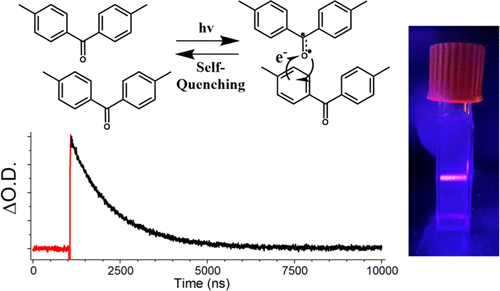
Kinetics of Photoreactive 4,4′-Dimethylbenzophenone Nanocrystals: Relative Contributions to Triplet Decay from Intermolecular H-Atom Transfer and Reductive Charge Transfer Quenching
Crystals of 4,4′-dimethylbenzophenone (DMBP) are known to react by intermolecular H atom transfer, followed by radical pair recombination. To determine the contribution of the H atom transfer reaction to the deactivation of the triplet ketone, transient absorption spectra and kinetics were obtained using aqueous nanocrystalline suspensions. Single-exponential lifetimes of ca. 1185 ns with no deuterium isotope effect and inefficient product formation suggest that the reaction does not contribute significantly to the kinetics of triplet decay. By contrast, the observed lifetime is consistent with previous observations with p,p′-disubstituted benzophenones that undergo an efficient self-quenching process by a reductive charge transfer mechanism.