Most Recent Publications
Topochemical Synthesis, micro-Electron Diffraction (3D ED), and Electron Beam-Initiated Topotactic Dimer Splitting of cis,syn-Thymine Dimer Crystals
A solid-state photochemical reaction of crystalline thymine hydrate (TH) resulted in a clean topochemical transformation into the cis-syn-dimer (TD), matching the structure as the one responsible for most UV lesions in DNA. Microcrystals of TD grown by drop casting piperidine solutions in a TEM grid made it possible to determine their structure by microelectron diffraction (3D ED) and to confirm expectations that an in situ electron-beam ionization reaction could result in a topotactic dimer splitting that, in this case, retains single-crystal-to-single-crystal character up to ca. 30% conversion. The packing structure of dimer TD and the as formed monomer T displays a novel trimeric hydrogen bonding motif, and the latter represents a new crystal phase. Beyond interesting analogies between single crystals of T and TD, and DNA, such as templated dimer formation and electron-transfer-induced repair, this work is a rare example of an electron beam-induced chemical reaction in the crystalline solid state.

J. Am. Chem. Soc. 2025, 147, 24, 20426–20430
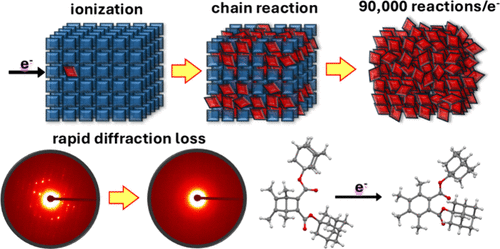
From Beam Damage to Massive Reaction Amplification under the Electron Microscope: An Ionization-Induced Chain Reaction in Crystals of a Dewar Benzene
Electron microscopy in its various forms is one of the most powerful imaging and structural elucidation methods in nanotechnology where sample information is generally limited by random chemical and structural damage. Here we show how a well-selected chemical probe can be used to transform indiscriminate chemical damage into clean chemical processes that can be used to characterize some aspects of the interactions between high-energy electron beams and soft organic matter. Crystals of a Dewar benzene exposed to a 300 keV electron beam facilitate a clean valence-bond isomerization radical-cation chain reaction where the number of chemical events per incident electron is amplified by a factor of up to ca. 90,000
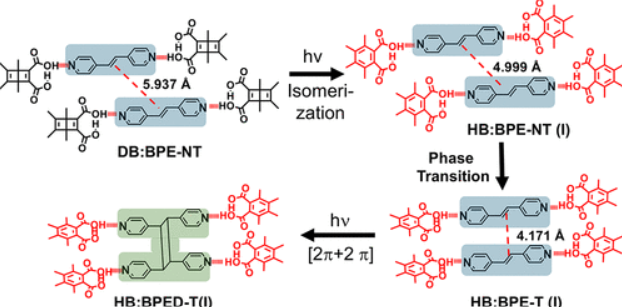
Cascade Photoreaction in Crystals: A Phase Change Caused by a Dewar Benzene Quantum Chain Triggers a Topochemical [2 + 2] Photodimerization
Crystals undergoing tandem reactions where the first transformation enables the second one are rare. Using photoreactive Dewar benzene 3,4,5,6-tetramethyl-1,2-dicarboxylic diacid (DB) as a hydrogen-bonding template for the [2π+2π] photodimerization of trans-4,4′-bipyridyl-ethenes (BPE), we obtained crystals DB-BPE-NT with a DB:BPE = 2:1 stoichiometry with double bonds at a nonreactive distance of 5.957 Å. Exposure to UV light resulted in valence bond isomerization to Hückel benzene 3,4,5,6-tetramethyl-1,2-dicarboxylic acid (HB) by a quantum chain reaction that triggered the sought-after topochemical [2π+2π] photodimerization reaction. Notably, crystals HB:BPE-T with the Hückel benzene isomer and BPE adopted a 2:2 stoichiometry and displayed an efficient topochemically templated photodimerization reaction